Return
to research document list...
|
Journal of Hepatology, vol. 37/1,pp 78-86, July 2002
Improved prognosis of postoperative hepatocellular carcinoma patients when treated with functional foods: a prospective cohort studyYoichi Matsui * matsui@takii.kmu.ac.jp , Junya Uhara, Sohei Satoi, Masaki Kaibori, Hitoshi Yamada, Hiroaki Kitade, Atsusi Imamura, Soichiro Takai, Yusai Kawaguchi, A.-Hon Kwon and Yasuo KamiyamaFirst Department of Surgery, Kansai Medical University, 10-15 Fumizono, Moriguchi, Osaka 570-8507, Japan AbstractBackground/Aims: Active hexose correlated compound (AHCC) is a newly developed functional food. In vitro experiments have shown that AHCC enhances natural killer cell activity, and may be considered a potent biological response modifier in the treatment of cancer patients. However, the effects of AHCC in a clinical setting have not been reported. We seek to determine whether AHCC can improve the prognosis of hepatocellular carcinoma (HCC) patients following surgical treatment.
Methods: A prospective cohort study was performed from February 1, 1992 to December 31, 2001. A total of 269 consecutive patients with histologically confirmed HCC were studied. All of the patients underwent resection of a liver tumor. Time to treatment failure (disease recurrence or death) and ten parameters related to liver function after surgery were examined.
Results: Of the 269 patients, 113 received AHCC orally after undergoing curative surgery (AHCC group). The AHCC group had a significantly longer no recurrence period (hazard ratio (HR), 0.639; 95% confidence interval (CI), 0.429-0.952; P=0.0277) and an increased overall survival rate (HR, 0.421; 95% CI, 0.253-0.701; P=0.0009) when compared to the control group by Cox's multivariate analysis.
Conclusions: This study suggests that AHCC intake can improve the prognosis of postoperative HCC patients. Keywords: Active hexose correlated compound; Biological response modifier; Cirrhosis; Functional food; Hepatitis; Hepatocellular carcinoma *Corresponding author. Tel.: +81-6-6992-1001, ext. 3262; fax: +81-6-6992-7343 1. IntroductionThe incidence of hepatocellular carcinoma (HCC) is distributed widely over different geographical areas. There is a high prevalence of HCC in Asia that is similar to that of stomach cancer in Japan. Moreover, the number of HCC patients is showing a gradual, but definite increase. The prevention and treatment of the recurrence of HCC following hepatic resection has been studied extensively. These treatments include repeated hepatic resection, interventional radiology (chemoembolization), percutaneous ethanol injections, percutaneous microwave coagulation, and the administration of hormonal agents. However, the prognosis for HCC remains unsatisfactory, with the 5-year survival rate after primary surgical treatment at approximately 40% in Japan. In addition to the treatments mentioned above, there have been many attempts to treat the cancer by stimulating the patient's immune system. Although several biological response modifiers (BRMs) have been developed such as BCG, Picibanil, PSK, lentinan, interferon, and interleukin-12, the clinical efficacy of these substances has not been clearly confirmed. Recently, the efficacy of immunotherapy in suppressing the postsurgical recurrence of HCC was reported as a clinical trial.
Active hexose correlated compound (AHCC) is a functional food developed by the Amino Up Chemical Co. Ltd (Sapporo, Japan) in 1989. A food is considered functional if it is satisfactorily demonstrated to affect beneficially one or more target functions in the body, in a way that is beyond adequate nutritional effects and is relevant to either the state of well-being and health or the reduction of the risk of a disease. The AHCC is an extract of Basidiomycotina, which is obtained through the hybridization of several types of mushrooms. Ghoneum et al. reported that AHCC enhances the natural killer (NK) cell activity of cancer patients, and may be considered a potent BRM in the treatment of cancer patients. It has been suggested that NK cell activity may be associated with cancer incidence. Furthermore, AHCC has been reported to reduce the metastasis rate of rat mammary adenocarcinomas, to increase detoxification enzymes in the liver, to protect the liver from CCl4-induced liver injury , and to prevent diabetes induced by streptozotocin in animal models. However, there have been no reports on the effects of AHCC in a clinical setting.
This study was initiated to evaluate the effects of AHCC, as an orally administered BRM, on the prognosis of patients with HCC following surgical treatment. 2. Patients and methodsTo determine whether AHCC can improve the prognosis of HCC patients following surgical treatment, a prospective cohort study was performed. All consecutive patients with HCC who underwent surgical treatment from February 1, 1992 to October 31, 2001 at the First Department of Surgery, Kansai Medical University, Osaka, Japan were included in this study, if they met the following criteria: (1) the patient had undergone a curative resection of their liver tumor at our Department and (2) the presence of histologically proven HCC in their resected liver specimen was demonstrated. The therapeutic options were offered to all of the patients during their hospitalization. The enrolled patients were addressed to each arm of the study based on their choice of the therapeutic options, and were trusted with the self-administration of AHCC. If the patient selected the AHCC ingestion, they began ingesting AHCC at 3.0g/day from the date of their discharge. The primary endpoint was survival and the secondary endpoint was a no recurrence period. In addition, ten biochemical parameters were examined yearly to evaluate the liver function until death or the end of the observation period (December 31, 2001). These parameters include the serum levels of aspartate transaminase activity (AST), alanine transaminase activity, alkaline phosphatase activity, g-glutamyltransferase activity (GGT), total bilirubin, albumin, cholinesterase activity, platelet count, a-fetoprotein, and protein induced by vitamin K absence (PIVKA II). Randomization was not performed in this study, and a placebo was not used for the controls. The study protocol conformed to the ethical guidelines of our institute and was approved by the institutional review committee. AHCC was generously provided by the Amino Up Chemical Co. Ltd, and was developed by extraction from a cultured broth of Basidiomycotina. 2.1. PatientsBy October 31, 2001, a total of 269 patients underwent surgical treatment for HCC. Of these 269 patients, a total of 47 cases were excluded as follows: 28 cases of non-curative resection, four cases of operative death, seven cases of hospital death, one case with a mental disorder, one case with primary biliary cirrhosis, one case with a histologically proven combined type of HCC and cholangiocellular carcinoma, and five cases that withdrew from follow-up just after discharge. As a result, the remaining 222 patients were enrolled in this study and were observed either until death or until the last follow-up date (December 31, 2001) for the living patients. Of these 222 patients, 113 were given AHCC (3.0g/day) orally after undergoing surgery, in accordance with the preferences of the patient (AHCC group). The administration of AHCC continued until death or to the last follow-up date for the living patients. The remaining 109 patients were monitored after the hepatectomy, but were not given AHCC (control group). The no recurrence rate and the overall survival of the patients in the AHCC group were compared to that of the control group. The aim of this study was explained to all of the approved patients in advance, and informed consent was obtained. All of the patients were trusted with their choice of AHCC ingestion, following the informed consent. Therefore, the patients were enrolled in either the AHCC group or the control group entirely according to their preferences. Although not a controlled study, we obtained a similar number of patients in each group with the same clinical and pathological characteristics according to the preferences of the patients. In the early years of the study, a few more patients preferred the control group than the AHCC group. However, the number of patients who preferred the functional food gradually increased. Eventually, approximately half of the patients preferred the functional food at the end of the study period of 9 years and 11 months. This change in preference resulted in the difference of the median follow-up period between the two groups shown in Section 3. 2.2. Follow-upPerioperative clinical parameters such as the patient characteristics, preoperative liver function data, operative factors, and tumor characteristics were compared between the AHCC and control groups. Cirrhotic status was histopathologically determined in non-cancerous liver tissues according to the New Inuyama Classification. The staging system used followed the General Rules for the Clinical and Pathological Study of Primary Liver Cancer by the Liver Cancer Study Group of Japan, which is commonly used in Japan. The overall survival, defined as the interval between the date of surgery and the date of death or the last follow-up information for the living patients, was also evaluated. The most common cause of death was cancer, but liver failure and variceal bleeding were included among the causes of death. The no recurrence rate was also evaluated, and was defined as the interval between the date of surgery to the date that a diagnosis of recurrence was confirmed by a positive sonogram, computed tomography, magnetic resonance imaging, or hepatic angiography. The no recurrence rate was calculated after censoring the patients who had not shown a recurrence at the time of death. All patients were given follow-up examinations with routine liver biochemical tests. Every 3 months, biochemical tests were performed at the central hospital laboratory. A liver ultrasound was also performed every 3 months. In addition, computed tomography and/or magnetic resonance imaging were performed every 6 months. Finally, an angiographic examination was performed after admission when a recurrence was suspected. Once an intrahepatic recurrence had been confirmed, patients in both groups generally received transarterial chemo-embolization (TACE), whereas some patients with recurrence underwent alternative treatments (Table 1). Patients without recurrence were not treated with any other drugs for cancer during follow-up. 2.3. Statistical analysisIn order to evaluate the homogeneity of the treatment vs. control groups with respect to perioperative clinical factors, data was analyzed using the chi-square test or Mann-Whitney U-test to compare differences between two series. A two-way analysis of variance with Scheff's F-test was used to compare the postoperative course of the laboratory data between the two groups. The no recurrence curves and the overall survival curves were plotted by the Kaplan-Meier method, and log-rank tests were also performed. The time-fixed Cox's proportional hazard model was used to estimate the effects of AHCC on the no recurrence rate and the overall survival. For univariate screening purposes, of the 40 potential risk factors shown in Table 1, the treatment for recurrence and the cause of death were excluded and the remaining 38 factors were examined univariately by the Cox's model, because these two variables were time-dependent factors. These 38 variables were all categorized as binary. Cirrhotic status was dichotomized into two categorical data group: with histopathologically confirmed cirrhosis or without histopathologically confirmed cirrhosis. The staging system was divided into two categorical groups: I/II and III/IVA. The resected liver volume was divided into two categorical groups: sub-segmentectomy or less and more than sub-segmentectomy. Since the median of the amount of alcohol intake was 0g/day of ethanol, the amount of alcohol intake was dichotomized into two groups: patients with a drinking habit and those without. The number of nodules was separated into two categorical data: single or not. The levels of tumor markers (a-fetoprotein and PIVKA II) that were highly skewed were divided into two categorical groups at 100µg/l and 100AU/l, respectively, which were clinically relevant. Other continuous variables were dichotomized into two groups at their overall median. All factors found to be significantly associated univariately with survival were included in the multivariate Cox's analysis with a stepwise method. The assumption of the proportional hazards was checked using the log-log plotting method and the parallel lines between the two groups were confirmed. A P-value of less than 0.05 was considered to be statistically significant. Table 1. Clinical background of patients treated with AHCC and controls a
a. AHCC, active hexose correlated compound;
TACE, transarterial chemoembolization; PEIT, percutaneous ethanol
injection therapy; PMCT, percutaneous microwave coagulation
therapy; ICG, indocyanine green; GSA, galactosyl human serum
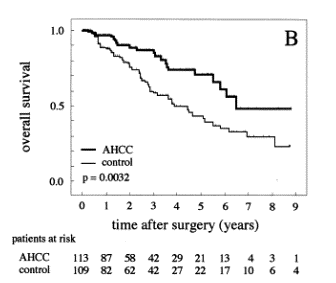
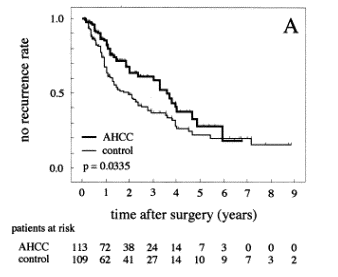
albumin; PIVKA, protein induced by vitamin K absence. 3. ResultsThe use of AHCC showed no side effects. Only three patients in the AHCC group refused to continue the use of AHCC during the study due to slight nausea. These three cases were censored at that time. Some cases had minor complaints of difficulty in swallowing the AHCC due to the granular type of its material. However, these patients did not stop the treatment. Four patients in the control group began to take AHCC during the observation period because they chose to take AHCC. These four cases were censored at that time. Table 1 demonstrates the similar clinical backgrounds of the patients between the two treatment groups. The albumin levels and the platelet count were significantly different between the two groups preoperatively. However, the differences were disadvantageous to the AHCC group. Most patients were diagnosed with an underlying viral hepatitis or cirrhosis, but they also had well-compensated liver function. No patients had ascites preoperatively. 3.1. No recurrence rate and overall survivalBy December 31, 2001, 39 (34.5%) patients had recurrences of HCC in the AHCC group, while 72 (66.1%) had recurrences in the control group. The results suggest that the use of AHCC had a significant effect (P=0.0335, log-rank test) on the no recurrence rate (Fig. 1A). Only 23 (20.4%) patients had died in the AHCC group by the end of the follow-up period, whereas 51 (46.8%) had died in the control group at the end of the follow-up period. The causes of death were 91.3% recurrence in the AHCC group and 92.2% in the control group. Patient survival was significantly higher (P=0.0032, log-rank test) in the AHCC group (Fig. 1B). The follow-up period ranged from 2 to 108 months in the AHCC group, and from 2 to 117 months in the control group. The median follow-up period was 28 months in the AHCC group and 30 months in the control group. Time-fixed Cox's univariate analysis was performed using all of the 38 variables mentioned above (Table 1). Of these 38 variables, the following 11 variables were significantly related univariately to the no recurrence rate: AHCC intake, cirrhosis, basal total bilirubin, basal cholinesterase activity, basal antithrombin III activity, ICG R 15min (%), blood transfusion, number of nodules, Stage, basal a-fetoprotein levels, and basal PIVKA II levels (Table 2). These variables were included in the Cox's multivariate analysis. In the last step, the following five variables entered the model and could not be removed: AHCC intake, basal total bilirubin, basal cholinesterase activity, number of nodules, and basal a-fetoprotein levels (Table 4). Accordingly, these five variables were significantly associated with the no recurrence rate, and were found to be independent factors. In the multivariate analysis, the hazard ratio of no recurrence in the AHCC group was reduced to 0.639 from 0.658 by the univariate analysis (Table 4).
Fig. 1. Kaplan-Meier estimates of the no recurrence rate and overall survival of HCC patients after hepatic resection. The thick line indicates survival in the AHCC group, and the thin line represents the control group. (A) No recurrence rate. There was a significant difference between the two groups on the log-rank test (P=0.0335). (B) Overall survival. There was also a significant difference between the two groups (P=0.0032). Of the 38 variables, the following 13 were significantly related univariately to the overall survival: AHCC intake, cirrhosis, Child classification, basal albumin level, basal cholinesterase activity, ICG 15min (%), ICG K value, operation length, number of nodules, blood transfusion, Stage, basal a-fetoprotein levels, and basal PIVKA II levels (Table 3). These 13 variables were included in the Cox's multivariate analysis. In the last step, the following five variables entered the model and could not be removed: AHCC intake, ICG 15min (%), number of nodules, Stage, and basal a-fetoprotein levels (Table 4). Accordingly, these five variables were significantly associated with the overall survival, and were found to be independent factors. In the multivariate analysis, the hazard ratio of overall survival in the AHCC group was reduced to 0.421 from 0.485 by the univariate analysis (Table 4). 3.2. Biochemical parametersTable 2. Significant variables in the univariate analysis for the no recurrence ratea
a. AHCC, active hexose correlated compound;
ICG, indocyanine green; PIVKA, protein induced by vitamin K
absence;ß, regression coefficients; SE,
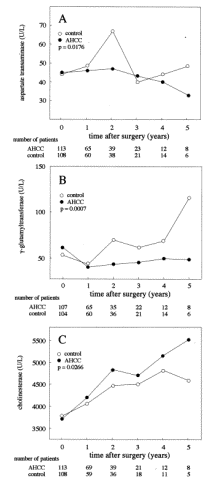
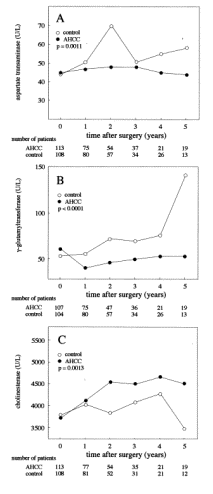
standard error; HR, hazard ratio; CI, confidence interval. 4. DiscussionHCC is a major health concern worldwide, with an incidence of approximately one million cases per year. Recently, the early detection of HCC has become possible because of progress in diagnostic imaging, and the incidence of resection for HCC has increased greatly during the last decade. As a result, the short-term outcome has improved greatly, and no-mortality series on liver resection for HCC were reported. Furthermore, there have been significant improvements in patients prognosis for those cases with HCC who were treated recently with liver resection in comparison to those treated with resection in the early 1990s . However, the long-term results are not yet satisfactory. Although hepatic resection is the most effective form of treatment for patients with HCC, the incidence of postoperative recurrence, which is the main cause of the poor long-term results, remains extremely high. Moreover, the cumulative intrahepatic recurrence rate has been reported at 100% at 5 years after the resection of a single HCC in cirrhotic patients with viral hepatitis. To prevent recurrence and/or to prolong survival, the most widely used option is adjuvant chemotherapy through a catheter inserted into the hepatic artery. However, the efficacy of these agents is very poor, the incidence of side effects is high, and there is no clear evidence suggesting that their administration results in improved survival . Furthermore, therapeutic doses of anti-cancer drugs have been reported to reduce the host anti-tumor immune response, and the postoperative use of immunosuppressants has been shown to accelerate the recurrence of malignancy. Thus, the search for other potentially useful therapeutic approaches is necessary. Recently, Takayama et al. reported some efficacy of adoptive immunotherapy on HCC recurrence on the basis of a randomized clinical trial. The remarkable results shown in this trial were very interesting and encouraging, although the procedure is relatively complicated and time-consuming, and requires hospitalization to perform. Under these circumstances, other options such as immunotherapy or radiation have little practical application in a daily clinical setting, and have been used only within research trials. The disappointing state of medical treatment for HCC justifies the interest in the administration of functional foods such as AHCC as a BRM, although its anti-tumor effects remain uncertain in a clinical setting. A major disadvantage in this study is that it is not randomized. A randomized trial would be of higher value, since the type of treatment allocation we have followed may prompt severe biases that are very difficult to control. However, most of the functional foods including AHCC, are on the market in Japan and are available easily without a prescription because it is not a medicine that is required to be prescribed by a physician. It is very difficult to strictly control the patients addressed to each arm, because the patients who prefer the functional food may obtain it out of the trial. Therefore, it is difficult to complete a randomized trial in regard to the functional foods. Allocation to each arm on the basis of the patient's own selection rather than randomization may be better for the trial of functional foods. In the case of the functional food, the patients enrolled may divide into each arm more exactly in the trial according to the preferences of the patients, compared to those in the randomized trial. Therefore, randomization was not considered in this study, despite many issues for severe biases in non-randomized trials. AHCC is an extract obtained from several species of mushrooms. AHCC contains various components, but the active component is an oligosaccharide with an average molecular weight of approximately 5000. Interestingly, in contrast to conventional active components such as the b-1,3-glucan structural component found in PSK and lentinan, the glucose oligomer in AHCC has an a-1,4-linkage structure and some esterified hydroxy groups. Therefore, AHCC may function as a BRM in the same manner as PSK and lentinan. In vitro experiments have shown that AHCC restores the NK cell activity that was depressed by an anti-cancer drug, and stimulated peritoneal macrophage cytotoxicity, NO production, and cytokine production. The combination of the anti-cancer drug and AHCC significantly improved the prognosis of mice after the excision of their primary tumors. Both NK cells and macrophages have been reported to be involved in the inhibition of tumor metastasis following activation by BRMs. Therefore, this AHCC effect may be mediated by the natural host immunity, which is restored or activated by AHCC. These findings suggest that AHCC may induce its therapeutic effects on the survival of HCC patients as a result of NK cell and macrophage activation. Accordingly, AHCC should be considered as a potent BRM, and its anti-cancer activity may be mediated through host immunomodulation. Recently, AHCC was reported to protect the liver from CCl4-induced liver injury in an animal model. The increased survival rate of the AHCC group suggests that AHCC may have had beneficial effects on the clinical course of patients with hepatitis or cirrhosis, in addition to its anti-cancer effects. Indeed, AHCC intake seemed to improve the hepatitis disease state, as suggested by improvements in the postoperative levels of AST and GGT reported here. In addition, the observation that the cholinesterase activity increased in the AHCC group suggests that AHCC intake results in some nutritional improvements in these patients. The analysis was also performed in patients who had no recurrence. This would eliminate the potential for a tumor related effect. Consequently, improvements of hepatitis and nutritional status in those who had no recurrence were also shown. These results show that the AHCC may improve liver function independent from the tumor. However, caution must be observed in interpreting the evaluation of these results. These biochemical parameters may improve because only those that survive can be examined. This eliminates those patients with poorer baseline profiles. In addition, if the control group has an undetected bias toward a worse outcome, the difference might be artificial. Our results suggest that the use of AHCC decreases both the probability of recurrence and the hazard of death due to HCC and/or liver cirrhosis. Furthermore, AHCC intake might also improve hepatitis or cirrhosis in the patients who had no HCC. The improvements in liver function in the AHCC group appear to reflect an improved prognosis, although a randomized-controlled trial is needed to confirm this observation if possible. The mechanisms responsible for the anti-cancer activity and/or the hepatitis-attenuating effect of AHCC were not explored in this study. At present, the effects of AHCC as the result of a single ingredient are difficult to explain, and it is similarly difficult to reach any conclusion regarding the complex effects of AHCC on patient survival. AHCC intake resulted in improved liver function, the prevention of recurrence of HCC after resection, and the prolonged survival of postoperative HCC patients without any adverse effects. Therefore, AHCC treatment is a valuable adjuvant therapy as a BRM in these patients. If possible, these observations need to be confirmed in larger, randomized-controlled double-blind trials. In addition, more detailed studies are required to elucidate the mechanisms responsible for the effects of AHCC. Table 3. Significant variables in the univariate analysis for the no recurrence ratea
a. AHCC, active hexose correlated compound;
ICG, indocyanine green; PIVKA, protein induced by vitamin K
absence; ß, regression coefficients; SE,
standard error; HR, hazard ratio; CI, confidence interval. Table 4. Significant variables in the univariate analysis for the no recurrence ratea
a. AHCC, active hexose correlated compound;
ICG, indocyanine green; PIVKA, protein induced by vitamin K
absence; ß, regression coefficients; SE,
standard error; HR, hazard ratio; CI, confidence interval.
AcknowledgementsWe thank Kohji Wakame and Kenichi Kosuna (Amino Up Chemical Co. Ltd) for providing the AHCC free of cost. ReferencesThe Liver Cancer Study Group of Japan, Primary liver cancer in Japan, In: S. Watanabe, S. Tominaga and T. Kakizoe, eds., Japanese Cancer Association. Cancer Treatment and Survival, Gann Monograph on Cancer Research No. 43 (1995) pages 81-95 (Japan Scientific Societies Press, Tokyo) M. Shimada, K. Takenaka, K. Taguchi, Y. Fujiwara, T. Gion, K. Kajiyama et al., Prognostic factors after repeat hepatectomy for recurrent hepatocellular carcinoma, Ann Surg 227 (1998) 80-85 M. Shimada, T. Matsumata, A. Taketomi, K. Yamamoto, H. Itasaka, K. Sugimachi, Repeat hepatectomy for recurrent hepatocellular carcinoma, Surgery 115 (1994) 703-706 J.L. Raoul, D. Guyader, J.F. Bretagne, J.F. Heautot, R. Duvauferrier, P. Bourguet et al., Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma, Hepatology 26 (1997) 1156-1161 P.E. Majno, R. Adam, H. Bismuth, D. Castaing, A. Ariche, J. Krissat et al., Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis, Ann Surg 226 (1997) 688-703 L. Castellano, M. Calandra, C.V. Blanco, I. De Sio, Predictive factors of survival and intrahepatic recurrence of hepatocellular carcinoma in cirrhosis after percutaneous ethanol injection: analysis of 71 patients, J Hepatol 27 (1997) 862-870 M. Ebara, M. Ohto, N. Sugiura, K. Kita, M. Yoshikawa, K. Okuda et al., Percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Study of 95 patients, J Gastroenterol Hepatol 5 (1990) 616-626 T. Seki, M. Wakabayashi, T. Nakagawa, T. Itho, T. Shiro, K. Kunieda et al., Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma, Cancer 74 (1994) 817-825 R. Murakami, S. Yoshimatu, Y. Yamashita, T. Matsukawa, M. Takahashi, K. Sagara, Treatment of hepatocellular carcinoma: value of percutaneous microwave coagulation, AJR 164 (1995) 1159-1164 E. Kouroumalis, P. Skordilis, K. Thermos, A. Vasilaki, J. Moschandrea, O.N. Manousos, Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study, Gut 42 (1998) 442-447 Cancer of the Liver Italian Programme Group, Tamoxifen in treatment of hepatocellular carcinoma: a randomized controlled trial, Lancet 352 (1998) 17-20 S. Pignata, B. Daniele, C. Gallo, R. De Vivo, S. Monfardini, F. Perrone, Endocrine treatment of hepatocellular carcinoma. Any evidence of benefit?, Eur J Cancer 34 (1998) 25-32 Y. Mizushima, N. Yuhki, M. Hosokawa, H. Kobayashi, Diminution of cyclophosphamide-induced suppression of antitumor immunity by an immunomodulator PS-K and combined therapeutic effects of PS-K and cyclophosphamide on transplanted tumor in rats, Cancer Res 42 (1982) 5176-5180 Y. Nishioka, M. Hirao, P.D. Robbins, M.T. Lotze, H. Tahara, Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12, Cancer Res 59 (1999) 4035-4041 J. Akiyama, T. Kawamura, E. Gotohda, Y. Yamada, M. Hosokawa, T. Kodama et al., Immunochemotherapy of transplanted KMT-17 tumor in WKA rats by combination of cyclophosphamide and immunostimulatory protein-bound polysaccharide isolated from basidiomycetes, Cancer Res 37 (1977) 3042-3045 H. Nakazato, A. Koike, S. Saji, N. Ogawa, J. Sakamoto, Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer, Lancet 343 (1994) 1122-1126 T. Takayama, T. Sekine, M. Makuuchi, S. Yamasaki, T. Kosuge, J. Yamamoto et al., Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial, Lancet 356 (2000) 802-807 J.A. Milner, Functional foods: the US perspective, Am J Clin Nutr 71 (Suppl) (2000) 1654S-1659S M.B. Roberfroid, Concepts and strategy of functional food science: the European perspective, Am J Clin Nutr 71 (Suppl) (2000) 1660S-1664S P.J. Agett, J. Alexander, M. Alles, P.A. Anderson, J.M. Antonie, M. Ashwell et al., Scientific concepts of functional foods in Europe consensus document, Br J Nutr 81 (1999) S1-S27 K. Matsushita, Y. Kuramitsu, Y. Ohiro, M. Obara, M. Kobayashi, Y.Q. Li et al., Combination therapy of active hexose correlated compound plus UFT significantly reduces the metastasis of rat mammary adenocarcinoma, Anticancer Drugs 9 (1998) 343-350 M. Ghoneum, Y. Ninomiya, M. Torabi, G. Gill, A. Wojdani, Active hemicellulose compound (AHCC) enhance NK cell activity of aged mice in vivo, FASEB J 6 (1992) A1213 K. Imai, S. Matsuyama, S. Miyake, K. Suga, K. Nakachi, Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population, Lancet 356 (2000) 1795-1799 B. Sun, K. Wakame, T. Mukoda, A. Toyoshima, T. Kanazawa, K. Kosuna, Protective effects of AHCC on carbon tetrachloride induced liver injury in mice, Nat Med 51 (1997) 310-315 K. Wakame, Protective effects of active hexose correlated compound (AHCC) on the onset of diabetes induced by storeptozotocin in the rat, Biomed Res 20 (1999) 145-152 F. Ichida, T. Tsuji, M. Omata, T. Ichida, K. Inoue, T. Kamimura et al., New Inuyama Classification: new criteria for histological assessment of chronic hepatitis, Int Hepatol Commun 6 (1996) 112-119 The Liver Cancer Study Group of Japan, The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 4th ed. edition, (Kanehara, Tokyo, 2000) R.G. Simonetti, A. Liberati, C. Angiolini, L. Pagliaro, Review. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials, Ann Oncol 8 (1997) 117-136 G. Torzilli, M. Makuuchi, K. Inoue, T. Takayama, Y. Sakamoto, Y. Sugawara et al., No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients, Arch Surg 134 (1999) 984-992 R.T.-P. Poon, S.-T. Fan, C.-M. Lo, I.O.-L. Ng, C.-L. Liu, C.-M. Lam et al., Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years, Ann Surg 234 (2001) 63-70 K. Shirabe, T. Kanematsu, T. Matsumata, E. Adachi, K. Akazawa, K. Sugimachi, Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses, Hepatology 14 (1991) 802-805 J. Belghiti, Y. Panis, O. Farges, J.P. Benhamou, F. Fekete, Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis, Ann Surg 214 (1991) 114-117 E. Mihich, Immnosuppression in cancer therapeutics, Transplant Proc 7 (1975) 275-278 © Copyright 2002, Elsevier Science, All rights reserved.
|