|
Dokkyo Journal of Medical Sciences
28(2-3). 745~752, 2001
Preventive Effects of Active Hexose Correlated
Compound
(AHCC) on Oxidative Stress Induced by Ferric
Nitrilotriacetate in the Rat
Shuyi Wang, Kaoru Ichimura and Koji Wakame *
Department of Biochemistry, Dokkyo University School of Medicine,
Mibu, Tochigi, 321- 0293 Japan and
* Research and Development Depal1ment, Amino UP Chemical Co Ltd.,
Sapporo, Hokkaido, 004- 0839 Japan
SUMMARY
Ferric nitrilotriacetate (Fe - NTA) is a strong oxidant, which generates
highly reactive hydroxyl radical and causes injuries of various
organs including the kidney and liver. The formation of 8- hydroxy
- 2' -deoxyguanosine (8- OHdG) adducts in the renal DNA is one of
the earliest events after treatment with Fe - NTA. Since Active
Hexose Correlated Compound (AHCC), an extract of fungi, has been
shown to act as an antioxidant, its protective effect on the oxidative
stress induced by Fe - NTA was examined in the present study. AHCC
at 3% in drinking water was given to male Wistar rats for 1 week,
then Fe - NTA was injected intraperitoneally. At 3 h after the treatment
with Fe - NTA, levels of 8- OHdG in the bladder urine, creatinine
in the serum, thymic apoptosis, serum levels of aspartate and alanine
aminotransferases were significantly increased.
All of these increases were restored to normal by
the AHCC pretreatment. These results suggest that AHCC is potent
in restoring the disorders of various organs induced by oxidative
stressors.
Key Words: ferric nitrilotriacetate, 8- hydroxy - 2' - deoxyguanosine,
creatinine, thymic apoptosis, aminotransferases, AHCC
INTRODUCTION
Reactive oxygen species (ROS) including superoxide (02- ), hydrogen
peroxide (H2O2), hydroxyl radical (. OH) are constantly generated
in aerobic respiration in response to both external and internal
stimuli). Low levels of ROS play an important role in signal transduction,
cell proliferation, apoptosis, immunity and defense mechanism 2-4.
High levels of ROS, however, cause severe metabolic malfunctions
and damage to biological macromolecules. For example, excess ROS
induce oxidative damages to nucleic acids and proteins, decrease
the efficiency of DNA polymerase repair and activity of signal transduction
and cause cell death by apoptosis or necrosis 2,5,6 . ROS induce
chemical changes in bases and change in DNA conformation, causimutation
4, 7. Damage to DNA by ROS is critical for carcinogenesis. Moreover,
the accumulation of free radical - mediated damage in DNA is the
major cause of the physiological changes associated with aging 8.
Animals including human possess numerous machineries of antioxidative
defense by themselves. Such defense mechanisms within the organism
have evolved to limit the levels of reactive oxidants and the damage
that they inflict 6. Enzymes involved in such mechanisms include
superoxide dismutase (SOD), glutathione peroxidase and catalase.
Moreover, there exist many structural defenses, for example, sequestration
of H2O2-generating enzymes in peroxisomes and chelation of any free
iron or copper salts in transferrin and ferritin or ceruloplasmin
to avoid the product of Fenton reaction, O2 -9 .
Epidemiological studies have suggested that antioxidative supplements,
such as dietary antioxidants of small molecules, including vitamin
C (ascorbate), vitamin E (tocopherol) and carotenoids are potent
in preventing carcinogenesis 10, 11. Thus, natural plant components
have been expected to provide oxidative defenses against cancer
and degenerative diseases caused by oxidative stress. Active Hexose
Correlated Compound (AHCC™ Amino UP Chemical Co. Ltd. Sapporo)
is a mixture of polysaccharides, amino acids and minerals derived
from fungi. It is obtained by hot water extraction after culturing
mycelia of several basidiomycetes in a liquid culture tank and treating
them with several enzymes 12. The main active component of AHCC
is a mixture of oligosaccharides whose average molecular weight
is approximately 5,000 12, 13. The chemical analysis has revealed
that polysaccharides are the major components of AHCC, consisting
pproximately 74%. Nearly 20% of ti1e polysaccharides are a- 1, 4-
glucan and its acetylated forms, which are thought to be active
components 12. AHCC also contains some other polysaccharides including
ß- glucans. Both a- and ß- glucans are shown to be responsible
for the antitumor effects of Basidiomycetes 14. It has been reported
that this extract shows various beneficial effects in humans and
in experimental animals acting either as a biological response modifier
(BRM) or an antioxidant. It was shown that AHCC was effective in
suppressing growth of experiment cancers in mice and rats such as
SST- 2 tumor and breast cancer l3. AHCC enhances a helper T cell
1 (Th1) response in susceptible BALB/c mice, speciaIly increasing
interleukin (IL) - 12 levels 15). It was also found out that Th1
cytokines levels such as tumor necrosis factor (TNF) - a and interferon
(IFN) - y were increased in response to AHCC treatment. AHCC is
used clinically as a successful complementary therapy for cancer
patients 16. AHCC treatment after surgical operation could improve
the quality of life (QOL) of patients with malignant tumors and
increase ti1e survival rate by inhibiting tumor metastasis 16. AHCC
can also prevent the onset of the diabetes induced by streptozotocin
17. Recently, we have found that AHCC suppresses thymic apoptosis
induced by dexamethasone 18 , suggesting that AHCC acts as an antioxidant.
Ferric nitrilotriacetate (Fe - NTA) is a chemical that induces severe
oxidative damage by Fenton reaction, in which the very reactive
hydroxyl radical is generated19~23. It acts as a potent nephrotoxin,
causing high incidence of renal cell carcinoma in rats and mice8,
20, 24 . Fe - NTA treatment results in renal DNA damage, including
formation of 8 - hydroxy - 2' - deoxyguanosine (8-OHdG) in the renal
DNA through the generation of active oxygen radicals. The increase
in urinary excretion of 8- OHdG reflects the oxidative DNA damage
in vivo 19, 25. Creatinine levels in the serum are significantly
increased following Fe - NTA treatment 19, 23i. Fe- NTA also can
induce hepatic injuries acting through the generation of R0S26.
These damages done to several organs are ameliorated by antioxidants27,
28.
The objective of our present study is to examine whether or not
AHCC has the preventive effects on the damage induced by oxidative
stress in rats treated with Fe- NTA. Our present study deals with
the beneficial effects of AHCC on the damage of various organs induced
by Fe - N1'A.
MATERIALS AND METHODS
1. Materials. Lyophilized AHCC was prepared by Amino UP Chemical
Co. Ltd. (Sapporo, Japan). Fe -NTA solution was prepared immediately
before use as previously described22). Nuclease P1 was obtained
from Sigma (St. Louis, MO, USA).
2. Animals. Studies with the rats were approved by Animal Care and
Use Committee, Dokkyo University School of Medicine. The animals
were treated according to guidelines for the Care and Use of Laboratory
Animals of ti1e Committee. Male Wistar rats of 8- weeks old were
purchased from Charles River Japan Inc. (Kanagawa. Japan). The animals
were kept in a room at 23 ± 2¼C with 12 h light and dark cycle and
kept free access to food and water.
Twenty- one rats were divided into four groups. Two groups received
3% AHCC as a drinking water for a week. The concentration of AHCC
and the duration of the treatment were chosen according to our previous
results 18. Control groups received only tap water for a week. Fe
- NTA (15 mg/kg body weight as Fe3 + was injected intraperitoneally
to two groups; one of them was AHCC - pretreatment group, and the
other was the control group. Fe - NTA effects at this dose were
maximal at 3h of the treatment as shown by others 27.28.
3. Blood sampling and organ preparation. Rats were sacrificed by
decapitation at 3h after Fe - NTA-treatment. Blood samples were
collected into sampling tubes. Serum samples were obtained after
centrifuging at 4¼C for 15 min at 3,000 rpm. The sera thus obtained
were used for the assay of serum aminotransferases and creatinine.
Thymus glands were removed and washed in cold phosphate - buffer
solution (PBS), then put into the tubes which contained collagenese-
EDTA for apoptosis assay. Urine samples were taken from urinary
bladder of rats for 8- OHdG assay.
4. 8- OHdG assay in the urine. Urine samples were centrifuged at
10,000 rpm, and the supernatant was used for ELISA analysis. 8-
OHdG was assayed by ELISA kits, which were prepared by Japan Institute
for the Control of Aging (Shizuoka, Japan) .
5. Detection of apoptosis by flow cytometry. Cell suspensions were
obtained after collagenase - EDTA (0.25% in PBS) treatment and filtration
through nylon mesh. The cell suspensions were washed with PBS (pH
7.4), adjusted to a concentration of 1.5 x 106 cell/mL.Then the
cells were centrifuged at 200 x g for 5 min. The 200 x g centrifuged
cell pellet was gently resuspended in1.5 mL hypotonic fluorochrome
solution containing propidium iodide (PI, 50 µg/mL in 0.1% sodium
citrate plus 0.1% Triton x 100) , in polypropylene tubes. The tubes
were placed at 4¼C in the dark overnight before the flow cytometric
analysis. Flow cytometry was carried out by analyzing 10,000 cells
per test using a FACSCalibur (Becton Dickinson, Mountain View, CA,
USA).
6. Creatinine and aminotransferase assays. Creatinine in the serum
was assayed by creatinine kit. The activity of alanine aminotransferase
(ALT) , aspartate aminotrans- ferase (AST) in the serum were routinely
assayed by commercial kits.
7. Statistical analysis. All the data were expressed as mean ± SEM.
Analysis of variance (ANOVA) was performed and Scheffe's multiple
comparison test was applied to test for the differences between
individual groups. A, p value less than 0.05 was considered statistically
significant.
RESULTS
1. 8- OHdG in the bladder urine First we observed the noxious effects
of Fe - NTA in the kidney and the effects of simultaneous administration
of Fe- NTA and AHCC on the renal function. 8- OHdG in the bladder
urine increased significantly at 3 h after treatment with Fe - NTA.
A significantly lower level of 8 - OHdG in the urine of AHCC pretreated
rats was observed, when compared with that of the rats treated with
Fe - NTA alone (Fig. 1).
2. Creatinine in the serum enhanced serum creatinine levels, which
depend on glomeruler filtration rate, are indicative of renal injury22.
23. In our experiment, the creatinine levels in the serum increased
significantly in the rats treated with Fe- NTA treatment alone,
while AHCC - pretreatment restored the increased levels to normal
(Fig. 2).
3. Thymic apoptosis Thymic apoptosis in the Fe- NTA treated rats
increased significantly compared with that in the control and AHCC
groups. Thymic apoptosis in the Fe- NTA -AHCC treated rats was much
lower than in the rats treated with Fe - NTA alone (Fig. 5).
4. Serum AST and ALT Hepatic injuries induced by Fe - NTA were assessed
by measuring the activity of serum aminotransferases. The serum
levels of AST and ALT were elevated in the Fe - NTA- treated group
than those in the control and AHCC groups (Figs. 3, 4). AHCC - pretreatment
decreased AST and ALT levels in the serum significantly to the normal
levels.
DISCUSSION
The results of the present study have shown that Fe -NTA increases
the urinary levels of 8- OHdG, and serum levels of creatinine, AST
and ALT. All these changes induced by Fe - NTA have already been
observed by others, showing that this toxin damages both the kidney
and the liver 19, 22. 23. In the present study it was first shown
that thymic apoptosis was induced by this chemical. These changes
may be closely associated with oxidative stress, because Fe - NTA
produces ROS in vivo. All the Fe - NTA - induced changes observed
here are restored to normal by the pretreatment with AHCC for 1
week. The results seem to suggest that AHCC can protect damages
of various organs caused by ROS.
Fe - NTA is a renal toxicant
and carcinogen in rats and mice 20, 24. This chemical increases
markedly the urinary excretion of 8- OHdG which is a useful marker
for measuring the level of oxidative D NA damage19, 25. The damage
in DNA is closely associated with aging and carcinogensis 8, 19,
20, 23, 24. A common form of DNA damage is the formation of hydroxylated
bases, which is considered to be an important event in carcinogenesis
induced by oxidative stress. The formation of 8- OhdG has been shown
to be suppressed by antioxidants such as vitamin E, vitamin C and
methionine 10, 11. Many other antioxidants have been shown to protect
the injuries induced by Fe - NTA. For example, N - acetylcysteine
(NAC), a precursor of intracellular cysteine and glutathione, prevents
renal damage induced by Fe - NTA in the rat 28. Alpha- tocopherol,
a lipid- soluble antioxidant, ameliorates renal proliferative response
and toxicity induced by Fe - NTA 27. AHCC decreased the Fe - NTA
- increased 8- OHdG levels in the urine, suggesting that this compound
prevents mutation by scavenging ROS.
It is reported that
Fe - NTA increases the hepatic ornithine decarboxylase (ODC) and
that the pretreatment of rats with butylhydroxytoluene (BHT), an
antioxidant, suppresses the increase in the enzyme activity26 .
Fe -NTA injected intraperitoneally into rats increasea plasma levels
of the two aminotransferases, ALT and AST and AHCC pretreatment
normalized the elevated enzyme activities. These findings suggest
that AHCC protect the hepatic damage induced by oxidative stress
of Fe - NTA.
Thymic apoptosis is
induced by glucocorticoids and oxidative stress and suppressed by
antioxidants such as melatonin30. Recently we have found that AHCC
suppresses thymic apoptosis induced by dexame-thasone 18. All these
findings suggest that AHCC acts as an antioxidant. It is not known
yet if AHCC acts directly as an antioxidant to scavenge ROS or induces
some enzymes, which cleavage ROS. It still remains unclarified which
components of AHCC are responsible for its protective effect against
oxidative stress. Our preliminary experiments have revealed that
AHCC itself has no antioxidant effect on thymocytes in vitro (data
not shown). The study is currently under way to test the effects
of individual a- and - ß glucans purified from AHCC.
Acknowledgments. The authors are thanks
for Prof. S. Matsuzaki, Department of Biochemistry -, Dokkyo University
School of Medicine for his critical review of our paper. Thanks
are also due to Amino UP Chemical Co. Ltd. for the supply of AHCC.
This study was supported in part by research grants from Tsukushi
Foundation.
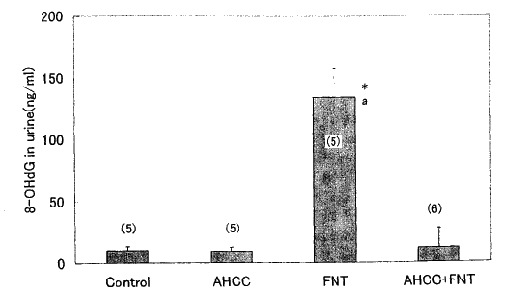
Fig. 1: Effects of Fe - NTA and AHCC on 8 -
OhdG levels in urinary bladder urine. Urine samples in the urinary
bladder were withdrawn immediately after sacrifice. Fe- NTA (15
mg Fe 3+/Kg) was injected i.p. 3h before experiment. AHCC at 3%
in drinking water was given for a week. Data are shown as means
± SEM with the numbers of determination in parentheses.
*, p< 0.001 vs. control group.
a, p< 0.001 vs. AHCC + Fe-NTA group.
Abbreviations are as follows: FNT, Fe-NTA, ferric nitrilotriacetate;
AHCC, Active Hexose Correlated Compound and 8 - OhdG, 8 - hydroxyl
- 2'- deoxyguanosine
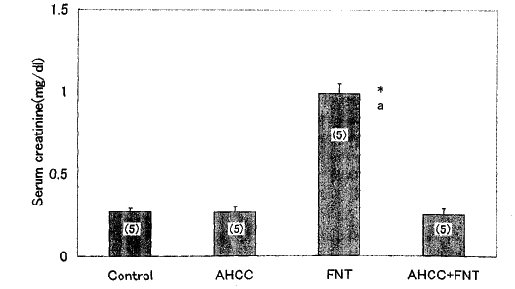
Fig. 2: Effects of Fe - NTA and AHCC on serum
creatinine levels. Creatinine in the serum were assayed by creatinine
kit. Data are shown as means ± SEM with the numbers of determination
in parentheses.

Fig. 3: Effects of Fe - NTA and AHCC on serum
alanine aminotransferase (ALT) levels.
ALT was assayed by commercial kit. Data are shown as means ± SEM
with the numbers
of determination in parentheses.
*, p< 0.001 vs. control group.
a, p< 0.001 vs. AHCC + Fe-NTA group.
Ê
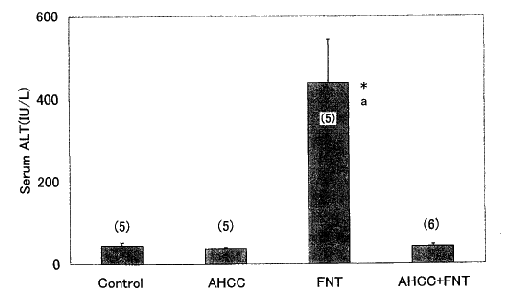
Fig. 4: Effects of Fe - NTA and AHCC on serum
aspartate aminotransferase (AST) levels.
AST was assayed by commercial kit. Data are shown as means ± SEM
with the numbers
of determination in parentheses.
*, p< 0.001 vs. control group.
a, p< 0.001 vs. AHCC + Fe-NTA group.
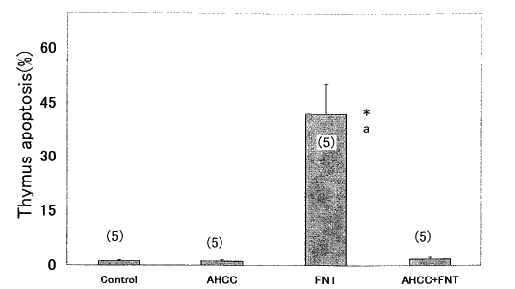
Fig. 5: Effects of Fe - NTA and AHCC on the
thymic apoptosis.
Apoptosis was estimated by flow cytometry using FACSCalibur. Data
are shown as
means ± SEM with the numbers of determination in parentheses.
*, p< 0.001 vs. control group.
a, p< 0.001 vs. AHCC + Fe-NTA group.
REFERENCES
1) Lee Y, Galoforo SS, Berns CM, et al: Glucose
deprivation - induced cytotoxicity and alterations in mitogen -
activated protein kinase activation are mediated by oxidative stress
in multidrug - resistant human breast carcinoma cells. J Biol Chem,
273: 5294- 5299, 1998.
2) Schulze - Osthoff K., Bauer MK, Vogt M, et
al: Oxidative stress and signal transduction. Int J Vitam Nutr Res,
67 : 336- 342, 1997.
3) Sun J, Chen Y, Li M et al: Role of antioxidant
enzymes on ionizing radiation resistance. Free Rad Biol Med, 24:
586- 593, 1998.
4) Vogt M, Bauer K, Ferrari K, et al : Oxidative
stress and hypoxia/reoxygenation trigger CD95 (APO- 1/Fas) ligand
expression in microglial cells. FEBS Lett, 429: 67- 72, 1998.
5) Baynes JW. : Role of oxidative stress in
development of complications in diabetes. Diabetes, 40: 405- 412,
1991.
6) Sies H.: Oxidative Stress: Oxidants and Antioxidants.
Academic Press, Orlando, Florida, 1991.
7) Burton K. A study of the conditions and mechanism
of the diphenylamine reaction for the colorimetric estimation of
deoxyribonucleic acid. Biochem J, 62 : 315- 323, 1956.
8) Harman D.: The aging process. Proc Natl Acad
Sci, USA, 78: 7124- 7128, 1981.
9) Biemond P, Van Eijk HG, Swak AJG, et al:
Iron mobilization from ferritin by superoxide derived from stimulated
polymorphonuclear leukocytes: possible mechanism in inflammation
diseases. J Clin Invest, 73: 1576- 1579, 1984.
10) Bendich A and Butterworth CE Jr.: Micronutrients
in Health and in Disease Prevention. Marcel Dekker, New York, 1991.
11) Gridley G, McLaughlin JK, Block G, et al: Vitamin
supplement use and reduced risk of oral and pharyngeal cancer. Am
J Epidemiol, 135: 1083- 1092, 1992.
12) Kosuna K.: Recent progress of research on AHCC.
New Food Industry, 41: 17- 23 (in Japanese), 1999.
13) Matsushita K, Kuramitsu Y, Ohiro Y, et al : Combination
therapy of Active Hexose Correlated Compound plus UFT significantly
reduces the metastasis of rat mammary adenocarcinoma. Anti -Cancer
Drugs, 9: 343- 350. 1998.
14) Mizuno T and Kawai M.: Chemistry and Biochemistry
of Mushroom (in Japanese). Japan Science Society Press, Tokyo, pp
35- 45, 1995.
15) Yagita A, Maruyama S and Sukegawa Y.: Effective
of the natural IL- 12 inducers on various progressive cancers (Abst).
AHCC Research Association 7th Symposium p. 15, 1999.
16) Matsui Y, Kawaguchi Y and Kamiyama Y.: Effects
of AHCC as a complemel1tary therapy on prognosis of postoperative
hepatocellular carcinoma patients (Abst). AHCC Research Association
7th Symposium, p. 16, 1999.
17) Wakame K.: Protective effects of Active Hexose
Correlated Compound (AHCC) on the onset of diabetes induced by streptozotocin
in the rat. Biomed. Res, 200 145- 152, 1999.
18) Burikhanov RB, Wakame K, Igarashi Y, et al: Suppressive
effect of Active Hexose Correlated Compound (AHCC) on thymic apoptosis
induced by dexamethasone in the rat. Endocrine Regulat, 34: 181-
188, 2000.
19) Kasai H.: Analysis of a form of oxidative DNA
damage, 8- hydroxy - 2' - deoxyguanosine, as a marker of cellular
oxidative stress during carcinogenesis. Mutation Res, 387: 147-
163,1997.
20) Okada S and Midorikawa S.: Induction of rats renal
adenocacinoma by ferric nitrilotriacetate. Jpn Arch Intenal Med,
29. 485- 491 (in Japanese), 1982.
21) Tanaka T, Nishiyama Y, Okada K, et al: Induction
and nuclear translocation of thioredoxin by oxidative damage in
the mouse kidney: independence of tubular necrosis and sulfhydryl
depletion. Lab Invest, 77: 145 - 55, 1997.
22) Toyokuni S, Uchida K, Okamoto K, et al: Formation
of 4- hydroxy - 2- nonenal - modified proteins in the renal proximal
tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate.
Proc Natl Acad Sci, USA. 91: 2616- 2620, 1994.
23) Umemura T, Sai K, Takagi A, et al: Formation of
8 - hydroxydeoxyguanosine (8- OHdG) in rat kidney DNA after intraperitoneal
administration of ferric nitrilotriacetate (Fe - NTA). Carcinogenesis.
11: 345- 347.1990.
24) Floyd RA. : 8- Hydroxy - 2' - deoxyguanosine in
carcinogenesis. Carcinogenesis, 11: 1447- 1450, 1990.
25) Shigenaga MK. Gimeno CJ and Arnes BN: Urinary
8 - hydroxy - 2' - deoxyguanosine as a biological marker of in vivo
oxidative DNA damage. Proc Natl Acad Sci, USA, 86: 9697- 9701, 1989.
26) Iqbal M, Giri U and Athar M.: Ferric nitrilotriacetate
is a potent hepatic tumor promoter and acts through the generation
of oxidative stress. Biochem Biophys Res Commun, 212: 557- 563,
1995.
27) Iqbal M, Rezazadeh H, Ansar S, et al: Alpha -Tocopherol
ameliorates ferric nitrilotriacetate (Fe -NTA) - dependent renal
proliferatjve response and toxicity: diminution of oxidative stress.
Hum Exptl Toxicol. 17:163- 171. 1998.
28) Umemura T, Hasegawa R. Sai- Kato K. et al : Prevention
by 2- mercaptoethane sulfonate and N -acetylcyteine of renal oxidative
damage in rats treated with ferric nitrilotriacetate. Jpn J Cancer
Res, 87: 882 - 886, 1996.
29) Gallagher SR.: Quantitation of DNA and RNA with
absorption and fluorescence spectroscopy. In: Ausubel I, Frederick
M (eds) Current Protocols in Molecular Biology. John Wiley &
Sons, New York, pp. A. 3D. 1 - A 3D.8, 1992.
30) Saimz RM. Mayo JC, Uria H, et al: The pineal neurohormone
melatonin prevents in vivo and in vitro apoptosis in thymocytes.
J Pineal Res, 19: 1 76- 188, 1995.
|





