Return
to research document list...
|
Protective Effects of Active Hexose Correlated Compound (AHCC) on the Onset of Diabetes Induced by Streptozotocin in the Rat
Koji Wakame, PhD
Department of Biochemistry, Dokkyo University School of Medicine, Mibu, 321-0293 Tochigi, and Research and Development Department, Amino UP Chemical Co. Ltd., Sapporo, 004-0839 Hokkaido, Japan
Biomedical Research 20 (3) 145-152, 1999 AbstractEffects of Active Hexose Correlated Compound (AHCC) on the onset of diabetes were studied in rats treated with Streptozotocin (STZ). AHCC was given to male rats at 4% in drinking water. A single i.v. injection of STZ (40mg/kg body weight) to rats resulted in an increase in blood glucose levels, a decrease in serum insulin levels, suppression of body weight gain, and an increase in serum GOT and GPT activities and serum levels of lipid peroxides. Treatment of AHCC restored these parameters to normal. Insulin immunoreactive B-cells in Langerhans islets reduced in number after treatment with STZ, while insulin immunoreactivity in the islets was normalized when AHCC was administered to STZ-treated rats. These results show that AHCC treatment is effective on the prevention of diabetes onset induced by STZ. Crude extracts derived from fungi of Basidiomycetes family such as lingzhi (Ganoder-malucidum, reishi) and zhuling (Polyporus umbellatus, chorei) are still used as components of Chinese traditional medicine. These fungi contain polysaccharides as well as other bioactive substances whose, actions include regulation of the immune system, antitumor action, hypo-glycemic effect, improvement of lipid metabolism and diuretic effect (26). Recent advances in culture techniques have enabled us to culture various species of Basidiomycetes. Active Hexose Correlated Compound (AHCC™, Amino UP Chemical Co; Ltd., Sappro) is a mixture of polysaccharides, amino acids and minerals derived from fungi. It is obtained by hot water extraction after culturing mycelia of several basidiomycetes in a liquid culture tank and then treating them with some enzymes. AHCC has been successfully used as a biological response modifier (BRM) in various disorders but only a little is known about its mechanism of actions. Recently we have reported that AHCC has hepatoprotective and detoxicating effects due to induction of hepatic enzymes and antioxidant action (23). It was also found that AHCC prolonged the life span and stimulated cytokine secretion from macrophages in mice bearing SST-2 tumor when treated together with 5-fluorouracil (5-FU)(14). Furthermore, it was shown that AHCC was effective in preventing lung metastasis in breast cancer-bearing mice when treated simultaneously with 5-FU (14). Sun et al. (13, 22) described that AHCC diminished the side effects caused by antitumor agents such as cyclophosphamide, 5-FU and cytosine arabinoside. In other words, the disorders in hematopoietic function, depilation and he-patotoxicity induced by these pharmaceuticals were all ameliorated by AHCC. These results suggest that AHCC restores the depression of the immune system and reduces side effects caused by antitumor agents, resulting in the prolongation of life span in experimental animals. It is reported that AHCC treatment for 2 years of patients with hepatoma who had undergone hepatectomy suppressed the remission and prolonged their life span significantly when compared with control patients (10). Yagita et al. (28) found that oral administration of AHCC to patients with malignant tumors resulted in an increase in serum levels of tumor necrosis factor (TNF)-a, interferon- g, and interleukin-12 and a decrease in serum immunosupressive acidic protein (IAP) and tumor growth factor (TGF)-b levels. AHCC has clinically shown to be beneficial for the treatment of other diseases. In addition to patients with malignant tumors (28), diabetic patients have been shown to respond to AHCC (11). Polysaccharides which lower elevated blood glucose levels were found in several fungi. For example, ganoderan A, B and C, polysaccharides derived from Ganoderma lucidum have hypo-glycemic activity in alloxan-treated mice (24). It is not known whether or not AHCC contain such anti-diabetogenic polysaccharides. In diabetes, the metabolism of glucose, proteins and lipids is abnormal due to the deficit in glucagon and insulin secretion, leading to various metabolic disorders (4) and onset of complications (8, 21). It is also reported that diabetics are highly sensitive to oxidative stress (1, 17, 25). The objective of the present study is to examine the preventive effects of AHCC on the onset of diabetes in rats treated with streptozotocin (STZ). This chemical is thought to induce type I diabetes possibly by stimulating xanthine oxidase (XOD) reaction in b-cells of Langerhans islets to increase O2 radicals, which in turn destroy the cells (9). Thus, the STZ-induced diabetes can be regarded as an experimental model of type I diabetes. The present study deals with the preventive effects of AHCC on the impairment of islet cells. Materials and MethodsMaterials: STZ was obtained from Sigma Chemical Co. (St. Louis, MO, USA). It was dissolved in 0.05 M citrate buffer, pH 4.5 at a Final concentration of 40 mg/mL. The solution was stored in darkness at 4C until use. Lyophilized AHCC was obtained from Amino UP Chemical Co. Ltd. (Sapporo, Japan). Animals: Male Wistar rats of 4 weeks old were purchased from Charles River Japan Inc. (Kanagawa, Japan). The animals were housed in a room kept at 22±2¡C with 12h light and dark cycle (light on 8 : 00-20 : 00 h) and kept free access to food and water. The general conditions of the rats were observed every day. The amount of water intake was measured by subtracting the amount of residual of water from that measured the previous day. A group of rats were given only AHCC at 4% in their drinking water for 3 weeks (AHCC-treated group). STZ at a dose of 40 mg per kg body weight was injected through the tail vein to 2 groups of rats (STZ-treated group). One of the STZ-treated groups received 4% AHCC in drinking water for one week prior to the treatment and for 2 weeks thereafter (STZ-AHCC treated group). Blood sampling and organ preparation. Blood was collected via the tail vein of the rats into EDTA-coated blood sampling tubes at 10:00 a.m. on the same day immediately after STZ treat-ment and at 7 and 14 days after STZ treatment. At 14 days after STZ treatment the rats were sacrificed by decapitation, and their livers and pancreases were removed. These organs were quickly rinsed in ice-cold saline solution. Pan creases were immediately fixed in 10% formalin. Biochemical analyses. Blood samples were centrifuged at 4¡C for 15 min at 3,000 rpm and the sera thus obtained were used for biochemical analyses. Serum GOT and GPT, blood glucose, lipid peroxides (LPO) and insulin were measured by Henry's method (5), glucose oxidase method, Yagi's method (27) and Insulin-EIA test (Wako, Osaka), respectively. Immunocytochemistry. The pancreatic tissue was fixed in 10% buffered formalin for 2 days, and embedded in parafin according to the conventional procedure. Paraffin sections were serially cut at 5 um in thickness and immumostained. Localization of insulin in pancreatic islets was visualized by the streptavidin-biotinylated horse-radish peroxiase complex immunoenzymatic technic using Histofine SAB-PO (M) kits (Nichirei, Tokyo, Japan) according to the protocol of the manufacturer. Briefly, a monoclonal antibody against rat insulin was employed as the first antibody. Reaction products were formed with 3, 3'-diaminobenzidine tetra-hydrochloride in the presence of hydrogen peroxide. The immumoreaction specifity was determined using normal serum instead of the specific antiserum. Statistical analysis. All the data were expressed as mean±SD. Statistical analysis was performed by Dunnett's multiple comparison test to control and non parametric method by Scheffe's multiple comparison test. A P value less than 0.05 was considered statistically significant. Results
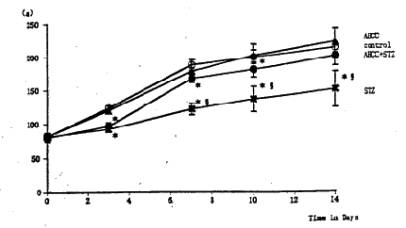
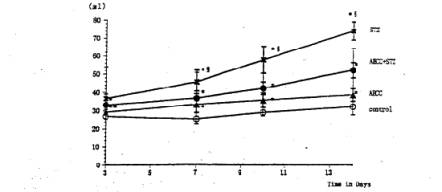
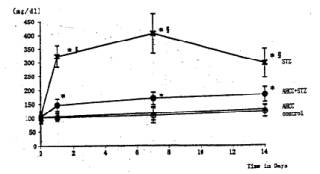
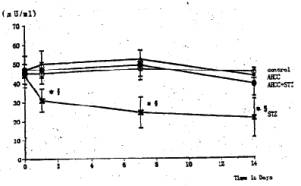
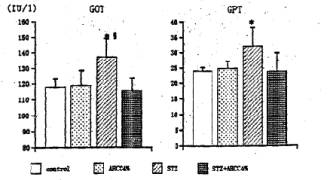
General Conditions Poor general conditions such as depilation and deterioration in quality of fur were noticed in the STZ-treated group. AHCC treatment resulted in an improvement of these conditions. AHCC alone showed no effect on body weight changes. A significant decrease in body weight gain was observed during 14 days after STZ treatment when compared with intact controls. The body weight gain of the STZ-AHCC treated rats was nearly the same as that of the intact control. Water intake An increase in the daily water consumption was noticed in the STZ-treated group (Fig. 3). AHCC given alone increased somewhat the water consumption but decreased the increased water intake when given to the STZ-treated rats. Biochemical analyses A significant rise in blood glucose levels was observed in the STZ-treated group up to 14th day after the drug treatment, reaching its maximum on 7 days after the treatment (Fig. 4). The blood glucose level at 14 days after STZ treatment was more than twice that in the control group. AHCC treatment nearly normalized the elevated levels of blood glucose in STZ-treated rats. The serum insulin levels decreased following STZ treatment and remained low till 14 days (Fig. 5). AHCC treatment restored the decreased insulin levels to normal. The serum levels of GOT, GPT and LPO were greater in the STZ-treated group than in the intact control and STZ-AHCC treated group at 14 days after the treatments. Histology of the pancreas A prominent decrease in the number of b-cells in the Islets of Langergans was observed in the STZ-treated group when compared with control group. The immunoreactivity of insulin in b-cells of the STZ- Paraffin sections obtained from the control and treated rats were immunostained with anti-insulin antibody. A prominent decrease in the number of insulin immunoreactive cells was recognized in STZ-treated group, while appreciable insulin cells were found in the STZ-AHCC treated group. DiscussionStreptozotocin (STZ) was originally discovered as a metabolite of Streptomyces achromogenes var. streptozotics by a research group in Upjohn Co. Ltd. in 1959. At first, there were expectations over possible clinical applications of its antibacterial and antitumor activities but it was soon found that diabetes was induced as a side-effect of STZ treatment. This chemical has widely been used as a diabetogenic agent there-after. Okamoto suggested that STZ induces diabetes through damaging DNA in the nuclei of pancreatic b-cells by alkylation, leading to an increase in poly (ADP-ribose) synthase. The increase in the enzyme activity results in a drastic decrease in nicotinamide adenine dinu-cleotide (NAD) concentrations of the yff-cells and then a decrease in the number of b-cells and death of the cells. All these changes may induce dysfunction of the pancreas. According to Kawada, STZ transported into b-cells through glucose transporter GLUT-2 located on their cell membranes is activated inside the cells and injures their mitochondria. This inevitably leads to a reduction of ATP generation through electron transport system and an increase in ADP concentrations. Subsequent degradation of ADP provides hypoxanthine, a substrate of xanthine oxidase (XOD). When XOD reaction takes place in the b-cells where XOD activity is intrinsically very high, O2 radicals are produced, resulting in cell damage and the onset of diabetes. STZ also activates XOD directly and augments 02" generation. The present study showed that the body weight of the STZ-treated rats decreased soon after STZ administration. This finding was in close agreement with that of other investigators (2, 3, 7, 12). In the STZ-AHCC treated group, however, no significant decrease in body weight was observed, suggesting that AHCC normalized the weight change caused by STZ. A significant increase in water intake, one of the general signs of diabetes, was observed in the STZ-treated rats. The water consumption was a little greater in the AHCC-treated groups than in the control, presumably because the rats had preference to AHCC-containing water which is rich in polysaccharides, amino acids, lipids and minerals. The water consumption of the STZ-treated group is much greater than that of the AHCC-treated groups. AHCC nearly normalized the water consumption and body weight gain of STZ-treated rats, suggesting that AHCC may have prevented the onset of diabetes. In the STZ-treated group, blood glucose rose immediately after the treatment and reached a quite high level at 14th day. The serum insulin levels decreased significantly in the STZ-treated group, indicating that their pancreatic b-cells were damaged. On the other hand, the blood glucose levels in the STZ-AHCC treated group were much lower than in STZ-treated group but slightly higher than in the control group. These results showed that mild diabetes did occur in the STZ-AHCC-treated rats, even though the serum insulin levels were restored to normal in STZ-AHCC treated rats. The serum levels of GOT, GPT and LPO increased in the STZ-treated group, while they were normalized by AHCC treatment. Rhee et ai (20) reported that the increase in the serum levels of GOT, GPT and LPO was due to the oxidative damage in the pancreas, liver, kidney and other organs caused by STZ through the increase in the free radical production. They suggested that the production of free radicals was augmented by the increase in arachidonate concentrations and the activity of lipoxygenase and cyclooxygenase. Furthermore, they observed the suppressive effects of antioxidants such as vitamin E on the onset of diabetes. The results of our present study suggest that AHCC suppresses the production of free radicals induced by STZ, whereby symptoms of diabetes were diminished. Diabetogenic substances such as STZ preferentially destroy nuclei of b-cells. Apoptosis has recently been suggested to be involved in STZ-induced degeneration of islet cells (15). Since b-cells of the islets decreased in number, one can expect that apoptosis may have occurred following STZ administration. The STZ-induced decrease in insulin immunoreactivity in islet b-cells was restored by AHCC. These findings suggest that AHCC prevents cellular damage induced by STZ and preserve the capability of insulin secretion. In conclusion, the results of our present study show that AHCC can prevent the onset of STZ-induced diabetes by protecting b-cells from degeneration and by diminishing oxidative injuries of cells in various organs. It remains to be clarified the mechanism by which AHCC acts as an antioxidant.
Fig. 1 Effects of AHCC and STZ on the body weight. Single intravenous injection of STZ reduced significantly the body weight gain. The body weight in the STZ-AHCC treated group was significantly larger than that in the STZ-treated group. Values are the means ±SD of 6 determinations in each group. * P<0.01 vs. control group. Ê P<0.01 vs. STZ-AHCC group.
Fig. 2 Effects of AHCC and STZ on water intake. Single intravenous injection of STZ caused significant increase in the water intake. Treatment of the diabetic rats with AHCC nearly normalized the water intake. The values are the means ±SD of 6 determinations in each group. * P<0.01 vs. control group. Ê ¤ P<0.01 vs. AHCC-STZ group.
Fig. 3 Effects of STZ and AHCC on serum glucose levels.. Single intravenous injection of STZ caused marked elevation of the blood glucose, reaching its maximum at 7 days of the treatment.. Treatment of the diabetic rats with AHCC nearly normalized the elevated serum glucose level. The values are the means ± SD of 6 determinations in each group. * P<0.01 vs. control group. P<0.01 vs. AHCC-STZ group.
Fig. 4 Effects of STZ and AHCC on serum insulin levels.. Single intravenous injection of STZ caused a significant decrease in the immunoreactive insulin level. AHCC restored the decreased serum insulin in diabetic with rats. Values are the means ± SD of 6 determinations in each group. * P<0.01 vs. control group. P<0.01 vs. AHCC-STZ group.
Fig. 5 Effects of STZ and AHCC on the activity of serum GOT and GPT. Rats were sacrificed at 14 days after STZ treatment and their sera were taken. The activity of the transaminases (GOT and GPT) was assayed as described in the text. Values are the means ± SD of 6 determinations in each group. * P<0.01 vs. control group. P<0.01 vs. AHCC-STZ group.
Table 1: Effects of STZ and AHCC on serum lipid peroxides (LPO)
* P<0.01 vs. control group. P<0.01 vs. AHCC-STZ group. References1. Baynes J.W. (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40, 405-412. 2. Craft N.E. and Failla M.L. (1983) Zinc, iron, and copper absorption in the streptozotocin-diabetic rat. Am. J. Physiol 244, E122-E128. 3. Failla M.L. and Kiser R.A. (1983) Altered tissue content and cytosol distribution of trace metals in experimental diabetes. J. Nutr. Ill, 1900-1909. 4. Genuth S.M. (1973) Plasma insulin and glucose profiles in normal, obese, and diabetic persons. Ann. Intern. Med. 79, 812-822. 5. Henry R.J.. Chiamori N.. Golub D;D. and Berkman S. (1960) Revised spectrophotometric methods for the deter-mination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am. J. din. Path. 34, 381-398. 6. Herr R.R., Eble T.E., Bergy M.F. and Jahnke H.K. Isolation and characterization of streotpzotocin (1960) Antibiot. Annual 1959-1960. 236-240. 7. Junod A.. Lambert A.E., Stauffacher W. and Renold A.E. (1969) Diabetogenic action ofstreptozotocin: relationship of dose to metabolic response. J. Clin. Invest. 48, 2129-2139. 8. Kannel W.B. and Mcgee. Dl (1979) Diabetes and cardiovascular disease. JAMA 241, 2035-2038. 9. Kawada J. (1992) New hypothesis for the mechanisms of streptozotocin and alloxan inducing diabetes mellitus. Yaktigaku Zasshi 112, 773-791 (in Japanese). 10. Kitade H., Matsui Y., Takai S., Imamura A., Kawaguchi Y., Kamiyama Y., Sun B. and Kosuna K. (1998) XXXIll-rd Congress of the European Society for Surgical Research, p.74 (Ab). 11. Kosuna K. (1999) Recent progress of research on AHCC (Active Hexose Correlated Compound), New Food Industry 41, 17-23 (in Japanese). 12. Luo W.Q., Kanno T., Winano A., Iwanaga T., Jun L., Futai Y., Yanahara C. and Yanahara N. (1998) An experimental analysis of therapeutic effects of a Chinese herbal prescription in streptozocin-treated rats. Biomed. Res. 19, 127-133. 13. Mukoda T., Sun B. and Kosuna K. (1998) Active Hexose Correlated Compound (AHCC) protects against cytosine arabinoside induced alopecia in the newborn rat animal model. Jpn. J. Cancer Res. 89, p.2405 (Ab). 14. Matsushita K., Kuramitsu Y., Ohiro Y., Obara M., Kobayashi M., Li Y.Q. and Hosokawa M. (1998) Combination therapy of Active Hexose Correlated Compound plus UFT significantly reduces the metastasis of rat mammary adenocarcinoma. Anti-Cancer Drugs 9, 343-350. 15. O'Brien B.A., Harmon B.V., Cameron D.P. and Allan D. J. (1997) Apoptosis is the mode of^-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes 46, 750-757. 16. Okamoto H. (1985) Molecular basis
of experimental 17. Prichard K.A., Patel S.T., Karpen C.W., Newman H.A.I. and Panganamala R.V. (1986) Triglyceride-lowering effect of dietary vitamin E in streptozotocin-induced diabetic rats. Diabetes 35, 278-281. 18. Rakieten N., Rakieten M.L. and Nadkarmi M.V. (1963) Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother. Rep. 29, 91-98. 19. Rerup C.C. (1970) Drugs producing diabetes through damage of the inslin secreting cells. Pharmacol. Rev. 22, 485-519. 20. Rhee S.J., Choe W.K. and Ha T.Y. (1995) The effect of vitamin E on the antioxidative defense mechanism in streptozotocin-induced diabetic rats. /. Jpn. Soc. Nutr. Food Sci 48, 451-457 (in Japanese). 21. Saudek C.D. and Eder H.A. (1979) Lipid metabolism in diabetes mellitus. Am. J. Med. 66, 843-852. 22. Sun B., Friedman M. and Kosuna K. (1998) Effect of AHCC (Active Hexose Correlated Compound) in both the prevention and treatment of carcinomas. Critical Appraisal of Unconventional I Alternative Interventions for Carcinoma of the Prostate. (Ab). 23. Sun B., Wakame K., Mukoda T., Toyoshima A., Kana-zawa T. and Kosuna K. (1997) Protective effects of AHCC on carbon tetrachloride induced liver injury in mice. Natural Med. 51, 310-315 (in Japanese). 24. Tomoda M., Gonda R., Kasahara Y. and Hikino H. (1986) Glycan structure of ganoderans B and C, hypo-glycemic glycans of Ganoderma lucidum fruit bodies. Phytochemistry 25, 2817-2820. 25. Urano S., Hoshi-Hashizume M., Tochigi N., Matsuo M., Shiraki M. and Ito H. (1991) Vitamin E and the susceptibility of erythrocytes and reconstituted liposomes to oxidative stress in aged diabetes. Lipids 26, 58-61. 26. Wagner H. (ed) (1999) Immunomodulatory Agents from Plants. Birkhauser Verlag, Basel, Boaton, Berlin. 27. Yagi K. (1976) A simple fluorometric assay for lipoperox-ide in blood plasm. Biochem. Med. 15, 212-216.
|